Research on drug quality under the international technical requirements of Hope
- Categories:Carrers
- Time of issue:2021-01-07
Research on drug quality under the international technical requirements of Hope
- Categories:Carrers
- Time of issue:2021-01-07
Spring rains, everything recovers!
On April 13, 2018, Beijing Hope Pharmaceutical Technology Co., Ltd. welcomed Dr. Tang Liya, founder and president of T3 PHARMA LINK, and Dr. Xiao Boming, general manager of Nanjing Baixian Pharmaceutical Technology Co., Ltd., to give guidance. He has published a wonderful academic report on drug quality research such as “Pharmaceutical Quality Regulations ICH and Analytical Methods” and “Starting, Endpoint and Cycle Management of Analytical Methods”.

Our company adheres to the policy of “taking technology as the core and talents as the foundation”, and continuously strengthens the scientific research and development of research and development personnel in the field of medical research. This academic report also injects advanced research into the company's chemical drug research and development work. R & D ideas, and through the case analysis of the company's projects, the theory and practice of drug development are perfectly combined.
In this training, Dr. Tang Liya directed the ICH aspect of the drug quality regulation: guidance on the specification and rationality of the drug analysis methods, especially the analytical methods at different stages of development; in addition to the pharmacopoeia standards and the CFDA's guidelines for drug development Control methods and requirements for various types of impurities, such as genotoxic impurities, isomers/chirals, and residual solvents. The starting point, end point and cycle management aspects of the analytical method: including the quality of the analytical method from the design, the method objectives, the risk assessment; the verification, validation and transfer of the analytical method. After the systematic training of the system, I believe that the ideas of colleagues in the development of analytical methods will be more clear in the future.

Dr. Tang explained the ICHQ8, Q9 and Q10 in detail. For drug research and development, the past focus on data transfer and variable output, but now more concerned with knowledge transfer and scientific/consistent output; quality risk management In the past, there was a lack of definition, unstructured, and now the use of structured processes; Q10 drug quality system, suggesting that in the future, we should establish a quality system throughout the product life cycle, and the entire life cycle quality system should be consistent; Q12 guides the enterprise In the operating procedures, it should be added beyond the specified limits, how to operate and deal with, to reduce the complexity of post-marketing changes, and provide new ideas for our research and development work.
Regarding genotoxic impurities, ICH M7 has detailed genotoxic impurity classifications, which are broadly classified into the following categories:
|
Impurity Class 杂质类别 |
Definition 定义 |
Guidance for control 控制指导 |
|
Class 1 |
Known mutagenic carcinogens 已知致突变致癌物 |
Control at or below compoundspecific acceptable limit 控制在化合物特异性的特接受限度以下 |
|
Class 2 |
Known mutagens with unknown carcinogenic potential (bacterial mutagenicity positive*, no rodent carcinogenicity data) 致癌性未知的已知致突变物(细菌致突变性阳性*,无啮齿动物致癌性数据) |
Control at or below acceptable limits (appropriate TTC) 控制在可接受限度(合适的TTC)以下 |
|
Class 3 |
Alerting structure, unrelated to the structure of the drug substance; no mutagenicity data 警示结构,与原料药结构无关;无致突变性数据 |
If non-mutagenic = Class 5 如无致突变性,归为5类 If mutagenic = Class 2 如有致突变性,归为2类 |
|
Class 4 |
Alerting structure, same alert in drug substance or compounds related to the drug substance 警示结构,与原料药结构有关;无致突变性数据 |
Q3A Q3B
|
|
Class 5 |
No structural alerts, or alerting structure with sufficient data to demonstrate lack of mutagenicity or carcinogenicity 无警示结构 |
The analytical techniques used in the analysis of genotoxic impurities are classified as follows:
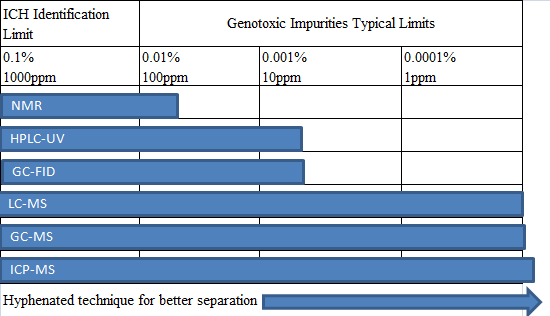
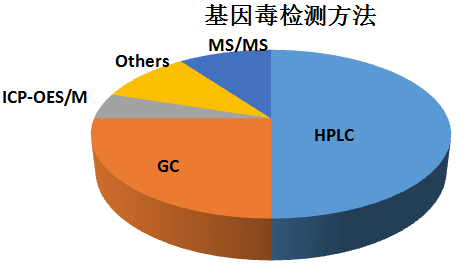
For most of the genotoxic impurities of the drug, LC-MS and GC-MS can be used for the detection. The percentages of the various analytical techniques are as follows:
For the quality of the analytical method from the design, Dr. Tang has a set of effective DOE methods. You can use the “fishbone diagram” or tabular form to analyze the risk factors of each parameter separately, and determine the analysis method design according to the size of the risk level. space.
Risk for Low or High Assay/Impurity Results(含量/有关物质风险评估表)
| Risk for Low or High Assay/Impurity Results(含量/有关物质风险评估表) | |||||
| Risk Factor (Failure mode) | Severity high=3 medium=2 low=1 |
Probability high=3 medium=2 low=1 |
Detectability high=1 medium=2 low=3 |
Numerical Rating S×P×D |
CQA |
| Environment | |||||
| Room Temperature | 1 | 1 | 1 | 1 | |
| Room Humidity(regular samples) | 1 | 2 | 1 | 2 | |
| Room Humidity(hydroscopic samples) | 3 | 2 | 1 | 6 | Y |
| Material | |||||
| sample | |||||
| Solvent(more risk on impurity) | 2 | 2 | 2 | 8 | Y |
| Water(more risk on impurity) | 2 | 2 | 2 | 8 | Y |
| Analyst | |||||
| Weighting | 3 | 2 | 2 | 12 | Y |
| Mixing | 3 | 2 | 2 | 12 | Y |
| Transferring | 3 | 2 | 2 | 12 | Y |
| Glassware | |||||
| Volumetric flask&Pipette | 3 | 2 | 1 | 6 | Y |
| Autosampler Vial (more risk on impurity) | 3 | 1 | 2 | 6 | Y |
| Bottle & Baker | 1 | 1 | 2 | 2 | |
Red represents high risk, yellow represents medium risk, and green represents low risk.
According to the results of the risk assessment and the results of the verification, the scheme of the transfer method of the analytical method is determined to make the scheme more scientific and reasonable.
Dr. Xiao Baiming gave a deeper comment and supplement to the lecture. At the same time, he re-intensified and consolidated the examples in the project, which enabled colleagues to have a deeper understanding of chemical drug research and development.
The lecture lasted for about 9 hours. Through this training, Hope Medicine's colleagues benefited a lot, and the advanced research and development ideas brought by the teachers opened up everyone's research and development vision. The two teachers also expressed their recognition of their attitudes and enthusiasm for learning. They believe that they can apply what they have learned in the future drug research and development work and contribute to the company's vigorous and steady development.
Beijing Hope Pharmaceutical Co., Ltd. 京ICP备17065299号




