【Good News】Haibu Pharmaceutical's Isavuconazole Sulfate API has obtained the CDE registration number.
Classification:
Company News
Release time:
2024-10-22

Basic information of raw materials
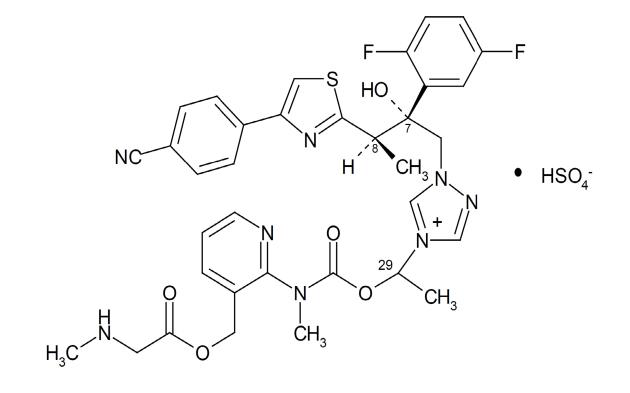
Generic name: Sulfate Isavuconazole
CAS NO.: 946075-13-4
Molecular formula: C35H35F2N8O5S·HSO4
Molecular weight: 814.84
Structural formula:
CAS NO.: 946075-13-4
Molecular formula: C35H35F2N8O5S·HSO4
Molecular weight: 814.84
Structural formula:
Product introduction
Isavuconazole was jointly developed by Astellas from Japan and Basilea Pharmaceutica from Switzerland. It is a triazole antifungal drug used for intravenous injection and oral administration; it has a broad antibacterial spectrum and is active against common pathogenic fungi including molds, yeasts, and dimorphic fungi.
On March 6, 2015, Isavuconazole was approved by the FDA for marketing to treat adult invasive aspergillosis and mucormycosis.
On December 14, 2021, Sulfate Isavuconazole capsules were approved by the NMPA for marketing, with the approved indications being: for the treatment of adult invasive aspergillosis and mucormycosis. It became the first oral antifungal drug approved in China for the treatment of adult invasive mucormycosis.
On June 20, 2022, injectable Sulfate Isavuconazole received marketing approval.
'Dosage form/Specification: Powder injection of 200mg; Capsule of 100mg'
'Patent status: Compound patent expired on October 25,2020'
'Contact us'
'Haibu Pharmaceutical has a rich variety of raw materials, welcome to inquire.'
Mr. Wei:18515385101;
Mr. Wu:15600038801
Beijing Haibu Pharmaceutical Technology Co., Ltd. (referred to as 'Haibu Pharmaceutical') was established in2005 and is a high-tech enterprise with 'chemical drug research and development' as its core capability. It has a Beijing R&D center, raw material drug and formulation pilot verification center,GMP industrial production base,
mainly engaged inthe technical development and technology transfer of chemical drugs, MAH research and holding certificates, raw material drug related declarations as well as commercial supply of pharmaceutical intermediatesand innovative and improved drug research declaration etc., has been awarded 'Top20 Chinese Pharmaceutical R&D Companies' for many consecutive years,and recognized as 'Beijing Science and Technology Research Development Institution', 'National High-tech Enterprise', 'Beijing Yizhuang Enterprise Innovation Center'.
Haibu Pharmaceutical has rich experience in the R&D transfer production, registration declaration etc. of various dosage forms such as chemical raw materials, oral solids, liquid preparations, sterile injections,and external preparations. The R&D products involve multiple indication areas such as anti-tumor, anti-depression, anti-epilepsy, anti-anxiety,cardiovascular system,digestive system,respiratory system.






