【Good News】Haibu Pharmaceutical's Riluzole raw material has obtained the FDA registration number in the United States.
Classification:
Company News
Release time:
2024-10-30

01. Basic Information of Raw Materials
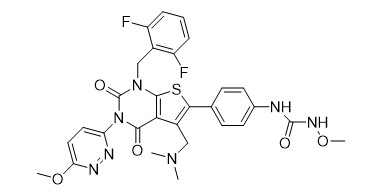
Chinese Name: Relugolix
English Name: Relugolix
CAS NO.: 737789-87-6
Molecular Formula: C29H27F2N7O5S
Molecular Weight: 623.63
Structural Formula:
02.Product Introduction
Relugolix is an oral gonadotropin-releasing hormone (GnRH) receptor antagonist that can block the binding of endogenous GnRH to its receptors, reducing the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby inhibiting the secretion of ovarian estradiol and progesterone, which can improve various symptoms caused by uterine fibroids. At the same time, the reduction in LH and FSH release can lower testosterone secretion, thus inhibiting the growth of prostate cancer cells.
On January 8, 2019, Takeda's Relugolix tablets (40mg) were approved for marketing by Japan's PMDA under the brand name Relumina for the treatment and symptom relief of uterine fibroids.
On December 18, 2020, Myovant Sciences' Relugolix tablets (120mg) were approved for marketing by the US FDA under the brand name Orgovyx as the first oral GnRH receptor antagonist for adult patients with advanced prostate cancer.
On May 26, 2021, the FDA approved a new drug from Myovant Sciences and Pfizer called Myfembree (each tablet contains Relugolix 40mg, Estradiol 1.0mg, Norethindrone Acetate 0.5mg), indicated for heavy bleeding related to uterine fibroids and pain caused by endometriosis.
Dosage Form/Specification: Tablets, 40mg/tablet, 120mg/tablet
Patent Status: Compound patent expires in January 2024
03. Contact Us
Our company has a rich variety of raw materials with stable supply; inquiries are welcome.
[Mr. Wei]:[Phone Number]; [Mr. Wu]:[Phone Number]






